Mastering Film-Forming Amines: Corrosion Control in Electrode Boilers
- Jan 19
- 5 min read
Abstract
Electrode boilers are increasingly used in industrial steam and heat generation as part of decarbonization strategies. Their operating principle differs fundamentally from conventional boilers and places new demands on water chemistry and corrosion control. This article explains how electrode boiler design influences corrosion mechanisms and shows, based on operational experience, how film-forming amines can be applied to stabilize operation, reduce iron transport, and protect critical components under flexible load conditions.
Introduction
Electric and electrode boilers have moved from pilot applications into regular industrial service. Rising shares of renewable electricity, volatile power prices, and the need for fast and flexible generation have made them an attractive option for steam production and district heating. Their ability to ramp quickly and operate efficiently at partial load fits well into modern energy systems.
At the same time, these advantages expose a weakness in traditional water chemistry concepts. In electrode boilers, water is no longer just a medium for heat transfer. It becomes an active part of the electrical circuit. As a result, conductivity, pH, oxygen control, and corrosion behavior are directly linked to electrical performance. This combination creates operating conditions that are fundamentally different from those in fossil-fired boilers.
Under flexible operation with frequent start-ups, shutdowns, and load changes, corrosion processes intensify. Iron transport increases, deposits accumulate, and standstill corrosion becomes a recurring issue. Conventional treatment programs, designed primarily for steady operation, often struggle to provide adequate protection under these conditions.
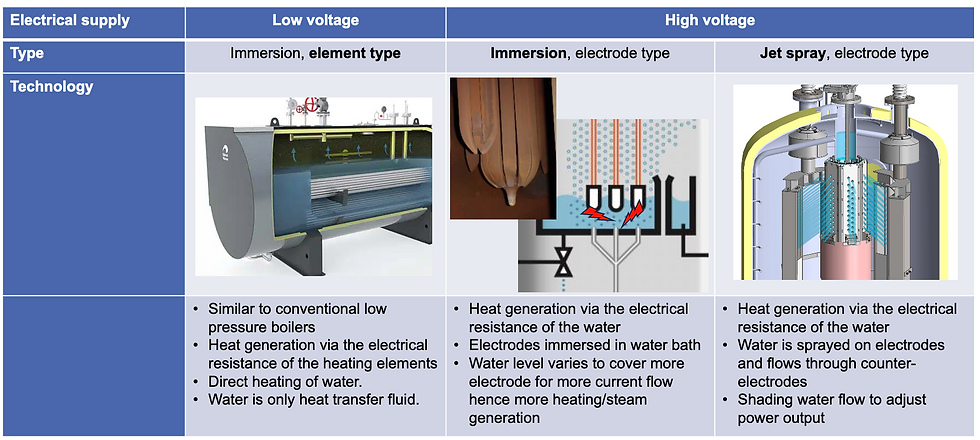
Electrode boiler design and its impact on corrosion
The corrosion behavior of electrode boilers is closely tied to their design. Low-voltage electric boilers, which use immersion heating elements, behave, from a water-chemistry perspective, much like conventional low-pressure boilers. Established standards apply, and the water functions purely as a heat carrier. Corrosion risks are familiar and manageable with proven treatment concepts.
High-voltage electrode boilers operate differently. Here, heat is generated by passing electrical current directly through the water. In immersion electrode designs, electrodes are submerged in a water bath and the electrical resistance of the water determines the load. This requires a precisely controlled conductivity window. At the same time, make-up water must be extremely pure to avoid uncontrolled changes in electrolyte chemistry.
Jet spray electrode boilers push this principle even further. Water is sprayed onto electrodes, creating very short contact times and requiring much higher conductivities. Although the demands on make-up water purity are less strict, the system still relies on stable chemistry to avoid corrosion, arcing, and material degradation.
In all high-voltage designs, water chemistry must satisfy two competing objectives. It must ensure stable electrical performance while simultaneously protecting metallic surfaces against corrosion. This inherent conflict is at the core of many operational problems.
Corrosion mechanisms under flexible operation
Electrode boilers are often operated in response to electricity market signals rather than process demand. As a result, they experience frequent load changes and extended idle periods. These conditions promote corrosion in several ways.
During low-load operation, iron release from electrodes and other components increases noticeably. When the boiler is shut down, residual moisture and oxygen trigger standstill corrosion almost immediately. High current densities can also promote side reactions at electrode surfaces, leading to hydrogen formation and accelerated material loss.
Over time, electrode wear alters the geometry of the system and affects operating parameters. Iron particles released into the water accumulate in filters, heat exchangers, and pumps, increasing maintenance effort and reducing availability. Traditional chemical treatment can control individual parameters such as conductivity or pH, but it rarely addresses surface protection during idle periods.
Film-forming amines as a protective concept
Film-forming amines have been used for decades in power plants and industrial boilers to control corrosion and protect systems during standstill. Their mode of action is based on adsorption onto metal and oxide surfaces, forming a thin, hydrophobic layer. This layer limits oxygen diffusion and stabilizes surface chemistry.
For electrode boilers, the application of film-forming amines requires particular care. Only products with fully disclosed composition and well-documented chemical properties should be used. High current densities and specific electrolyte conditions make it essential to understand how the product behaves under electrical stress.
Monitoring plays a central role. Residual concentrations must be measured reliably, and water chemistry parameters need to be interpreted in the context of film formation and potential decomposition products. When these conditions are met, film-forming amines can complement existing treatment concepts without interfering with electrical performance.
Industrial steam generation in New Zealand
One of the first documented applications of film-forming amines in an electrode boiler took place in New Zealand. An industrial steam generator was installed to replace a coal-fired boiler supplying steam for wool washing and drying. The new high-voltage immersion electrode boiler operated at 8 MW and required tightly controlled water chemistry.
During commissioning, unexpected problems emerged. A malfunctioning deaerator led to elevated oxygen levels, and severe corrosion followed. Boiler water turned black, iron concentrations increased sharply, and filters and analyzers became clogged. The situation posed a serious risk to the long-term viability of the installation.
After extensive discussion with the boiler manufacturer, a film-forming amine was introduced during the extended commissioning phase. A modified boil-out procedure with elevated concentration was used to establish surface protection quickly. Once normal operation began, the dosing was reduced to a stable residual level.
The effect was immediate and measurable. Iron concentrations dropped rapidly, boiler water clarity improved, and inspections revealed hydrophobic surfaces throughout the system. Importantly, no negative impact on electrical performance or hydrogen formation was observed. The boiler could operate stably under flexible conditions with significantly reduced corrosion activity.

District heating applications in Finland
Further experience was gained in Finland, where large electrode boilers are used for district heating. Two installations, rated at 40 MW and 50 MW, showed a continuous increase in iron concentration during operation. To control this, operators were forced to drain and refill the systems repeatedly, increasing water consumption and maintenance effort.
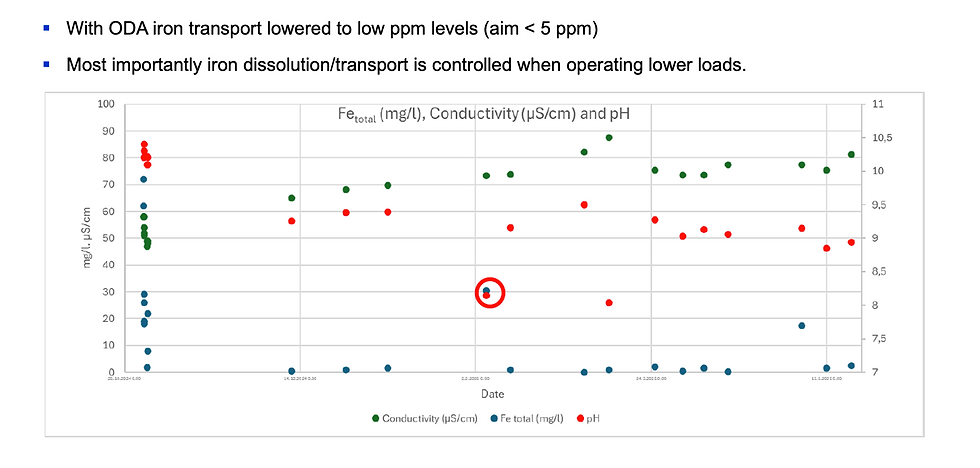
Film-forming amines were introduced with a conservative dosing strategy. Residual concentrations were maintained at low levels and replenished periodically to compensate for decomposition caused by high current densities. Blowdown was used to remove accumulated solids from the bottom of the boilers.
Within weeks, iron concentrations decreased significantly and stabilized at low levels. Conductivity and pH remained stable, and the boiler water became visibly clearer. Over time, inspections showed reduced electrode wear, particularly during low-load operation. An additional benefit was improved filtration efficiency, as iron particles formed larger agglomerates that could be removed more easily.
Operational insights
Experience from these applications highlights several recurring patterns. Iron release is highest during low-load operation and immediately after start-up. Standstill corrosion plays a major role in overall material loss. Film-forming amines reduce both effects by stabilizing surfaces and maintaining protection even during idle periods.
At the same time, application concepts must be tailored carefully. Decomposition under high current densities, interactions with electrolyte chemistry, and system-specific operating regimes all influence dosing strategies. Continuous monitoring and periodic inspection remain essential.
Conclusion
Electrode boilers introduce a new set of challenges for water chemistry and corrosion control. Their reliance on water as both an electrical and thermal medium creates conditions that traditional treatment programs were not designed to handle.
Operational experience shows that film-forming amines can provide an effective additional layer of protection. When applied with full understanding of the system and supported by careful monitoring, they reduce iron transport, stabilize operation under flexible load conditions, and extend component lifetime without compromising electrical performance.
As electrode boilers continue to gain relevance in industrial and district heating applications, film-forming amines are likely to become an integral part of future water chemistry concepts.
Author
Bio Ronny Wagner is the Managing Director at REICON Wärmetechnik und Wasserchemie Leipzig GmbH. As an experienced water treatment professional, he specializes in the application of film-forming amines in water-steam cycles, as well as in closed cooling and heating systems. With over 15 years of experience in the preservation of nuclear, fossil, and industrial power plants, he has played a pivotal role in advancing industry best practices. As an active member of vgbe and the IAPWS Power Cycle Chemistry (PCC) group, he has co-authored several international standards for the safe and effective application of film-forming amines in power plant chemistry.


